The human body is a miraculous, well-oiled, and exceptionally complex machine. It requires a multitude of functioning parts to come together for a person to live a healthy life—and every biological detail in our bodies, from the mundane to the most magical, is driven by just 21 chemical elements. Of the 118 elements on Earth, just 21 of them are found in the human body. Together, they make up the medley of divergent molecules that combine to form our DNA, cells, tissues, and organs. Based on data presented by the International Commission on Radiological Protection (ICRP), in the above infographic, we have broken down a human body to its elemental composition and the percentages in which they exist. These 21 elements can be categorized into three major blocks depending on the amount found in a human body, the main building block (4 elements), essential minerals (8 elements), and trace elements (9 elements).
The Elemental Four: Ingredients for Life
Four elements, namely, oxygen, carbon, hydrogen, and nitrogen, are considered the most essential elements found in our body. Oxygen is the most abundant element in the human body, accounting for approximately 61% of a person’s mass. Given that around 60-70% of the body is water, it is no surprise that oxygen and hydrogen are two of the body’s most abundantly found chemical elements. Along with carbon and nitrogen, these elements combine for 96% of the body’s mass. Here is a look at the composition of the four elements of life: Values are for an average human body weighing 70 kg. Let’s take a look at how each of these four chemical elements contributes to the thriving functionality of our body:
Oxygen
Oxygen plays a critical role in the body’s metabolism, respiration, and cellular oxygenation. Oxygen is also found in every significant organic molecule in the body, including proteins, carbohydrates, fats, and nucleic acids. It is a substantial component of everything from our cells and blood to our cerebral and spinal fluid.
Carbon
Carbon is the most crucial structural element and the reason we are known as carbon-based life forms. It is the basic building block required to form proteins, carbohydrates, and fats. Breaking carbon bonds in carbohydrates and proteins is our primary energy source.
Hydrogen
Hydrogen, the most abundantly found chemical element in the universe, is present in all bodily fluids, allowing the toxins and waste to be transported and eliminated. With the help of hydrogen, joints in our body remain lubricated and able to perform their functions. Hydrogen is also said to have anti-inflammatory and antioxidant properties, helping improve muscle function.
Nitrogen
An essential component of amino acids used to build peptides and proteins is nitrogen. It is also an integral component of the nucleic acids DNA and RNA, the chemical backbone of our genetic information and genealogy.
Essential and Supplemental Minerals
Essential minerals are important for your body to stay healthy. Your body uses minerals for several processes, including keeping your bones, muscles, heart, and brain working properly. Minerals also control beneficial enzyme and hormone production. Minerals like calcium are a significant component of our bones and are required for bone growth and development, along with muscle contractions. Phosphorus contributes to bone and tooth strength and is vital to metabolizing energy. Here is a look at the elemental composition of essential minerals: Values are for an average human body weighing 70 kg. Other macro-minerals like magnesium, potassium, iron, and sodium are essential for cell-to-cell communications, like electric transmissions that generate nerve impulses or heart rhythms, and are necessary for maintaining thyroid and bone health. Excessive deficiency of any of these minerals can cause various disorders in your body. Most humans receive these minerals as a part of their daily diet, including vegetables, meat, legumes, and fruits. In case of deficiencies, though, these minerals are also prescribed as supplements.
Biological Composition of Trace Elements
Trace elements or trace metals are small amounts of minerals found in living tissues. Some of them are known to be nutritionally essential, while others may be considered to be nonessential. They are usually in minimal quantities in our body and make up only 1% of our mass. Paramount among these are trace elements such as zinc, copper, manganese, and fluorine. Zinc works as a first responder against infections and thereby improves infection resistance, while balancing the immune response. Here is the distribution of trace elements in our body: Values are for an average human body weighing 70 kg. Even though only it’s found in trace quantities, copper is instrumental in forming red blood cells and keeping nerve cells healthy. It also helps form collagen, a crucial part of bones and connective tissue. Even with constant research and studies performed to thoroughly understand these trace elements’ uses and benefits, scientists and researchers are constantly making new discoveries. For example, recent research shows that some of these trace elements could be used to cure and fight chronic and debilitating diseases ranging from ischemia to cancer, cardiovascular disease, and hypertension. on They can take many forms, from the venom of a snake or spider to the neurotoxins produced by certain types of algae or microbes. In the infographic above, we look at some common biotoxins in the natural world and rank them based on how deadly they are to an average 70 kg (154 lb) human being.
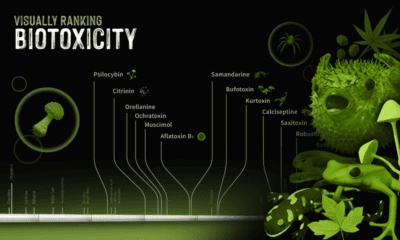
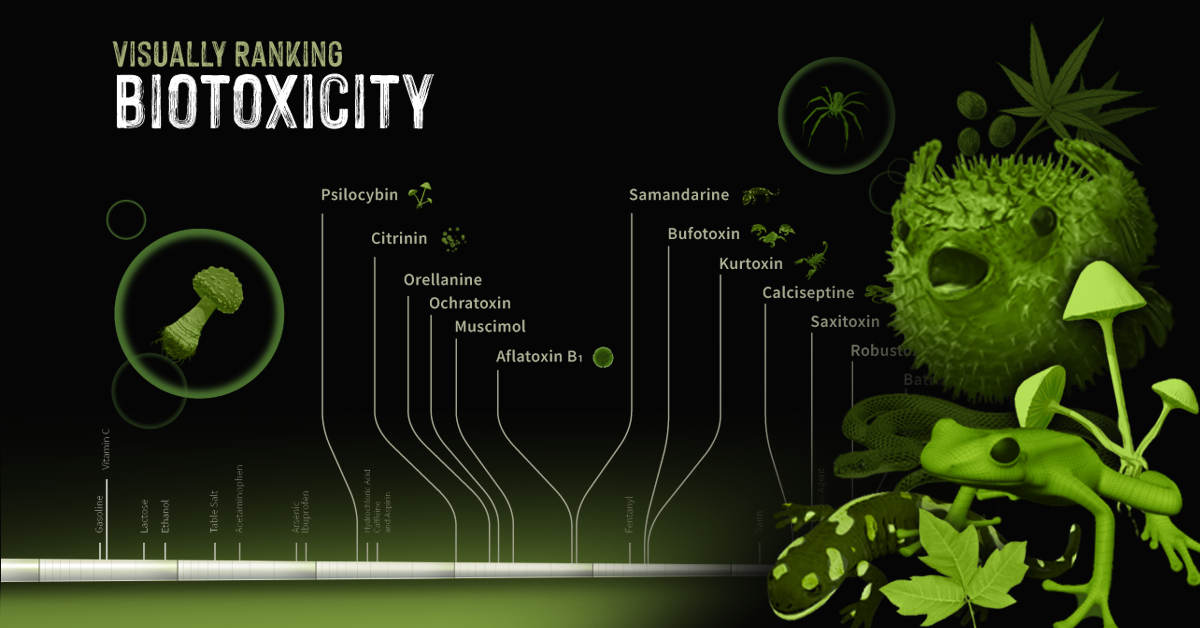
Ranking Biotoxins on a Toxic Scale
A basic concept in toxicology is that “only the dose makes the poison”. Everyday harmless substances like water have the potential to be lethal when consumed in large enough concentrations. Measuring a lethal dosage is very difficult. First, living things are complex: factors like size, diet, biochemistry, and genetics vary across species. This makes it difficult to qualify toxicity in a universal way. Second, individual factors like age or sex can also affect how deadly a substance is. This is why children have different doses for medications than adults. Third, how a poison is taken into the body (orally, intravenously, dermally, etc.) can also impact its deadliness. As a result, there are many ways to measure and rank toxicity, depending on what substance or organism is under investigation. Median lethal dose (LD50) is one common way for measuring toxicity. LD50 is the dose of a substance that kills 50% of a test population of animals. It is commonly reported as mass of substance per unit of body weight (mg/kg or g/kg). In the graphic above, we curate LD50 data of some select biotoxins found in nature and present them on a scale of logarithmic LD50 values. What’s surprising is just how potent some toxins can be.
Bits and Bites about Biotoxins
While one would think that biotoxins are avoided at all costs by humans, the reality is more complicated. Here are some interesting facts about biotoxins present in nature, and our unusual relationships with the organisms that create them:
- Fungi and molds make poisons called mycotoxins Mycotoxins are a global problem. They affect crops from many countries, and can cause significant economic losses for farmers and food producers.
- Phytotoxins can defend plants…and attack cancer Plants use phytotoxins to defend themselves other organisms, like humans. Urushiol, for example, is the main toxic component in the leaves of poison ivy, poison oak, and sumac. But the Pacific yew tree produces taxol that’s valuable in chemotherapy treatments.
- Fire salamander toxin is an ingredient in Slovenian whisky Though not widely available, some whisky makers in Slovenia use samandarine from the fire salamander to create a psychedelic alcohol.
- Ciguatoxins exist in the guts of reef fish Very unique species of bacteria living in the digestive tract of reef fishes make ciguatoxin. They transmit this poison to other organisms when the host fish is eaten.
- Pufferfish are deadly, but also delicious Pufferfish contain tetrodotoxin, a potent neurotoxin in their ovaries, liver, and skin called tetrodotoxin. Despite being a delicacy in many countries around the world, it has a lot of strict regulations because of its ability to kill people. In Japan, for example, only specially licensed chefs can prepare pufferfish for consumption.
- Batrachotoxin is lethal to the touch The skin of some poison dart frogs secretes a deadly substance called batrachotoxin. It is so potent that simply touching the poison can be fatal. Indigenous people of Central and South America used batrachotoxin to poison the tips of hunting weapons for centuries.
- Botox contains the most deadly biotoxin known Commercial botox uses an extremely small amount of biotoxin from a microbe called Clostridium botulinum. It paralyzes the muscles, preventing contraction (i.e. wrinkling). It is the deadliest known biotoxin on Earth. One gram of botulinum toxin can kill up to one million people.
Caveats of Measuring and Reporting Biotoxicity
While we use LD50 data to rank biotoxicity, it isn’t an exact science. There is room for improvement. For starters, no LD50 data exists for humans. That means data from other organisms has to be converted to apply to humans. There is a lot of contention amongst scientific communities about how accurate this is. There has also been an increasing effort to move to new methods of measuring toxicity that are not harmful to animals. Several countries, including the UK, have taken steps to ban the oral LD50, and the Organisation for Economic Co-operation and Development (OECD) abolished the requirement for the oral test in 2001. Now, new ways of evaluating toxicity are under investigation, like cell-based screening methods. Correction: Water was mislabeled on a previous version of the infographic. Full sources here